
Technical Products (TPs) on norms and standards, data and research
Technical Products (TPs) on norms and standards, data and research, was adopted as one of the strategic shifts under PB 22-23. The TP initiative has been built enhancing lessons from the Global Public Health Goods planned for PB 20-21. In the context of WHO transformation and guided by GPW13, TPs are products that are applicable to multiple countries and developed through rigorous processes at global, regional and country level to drive impact, detailed in the TP Guide.
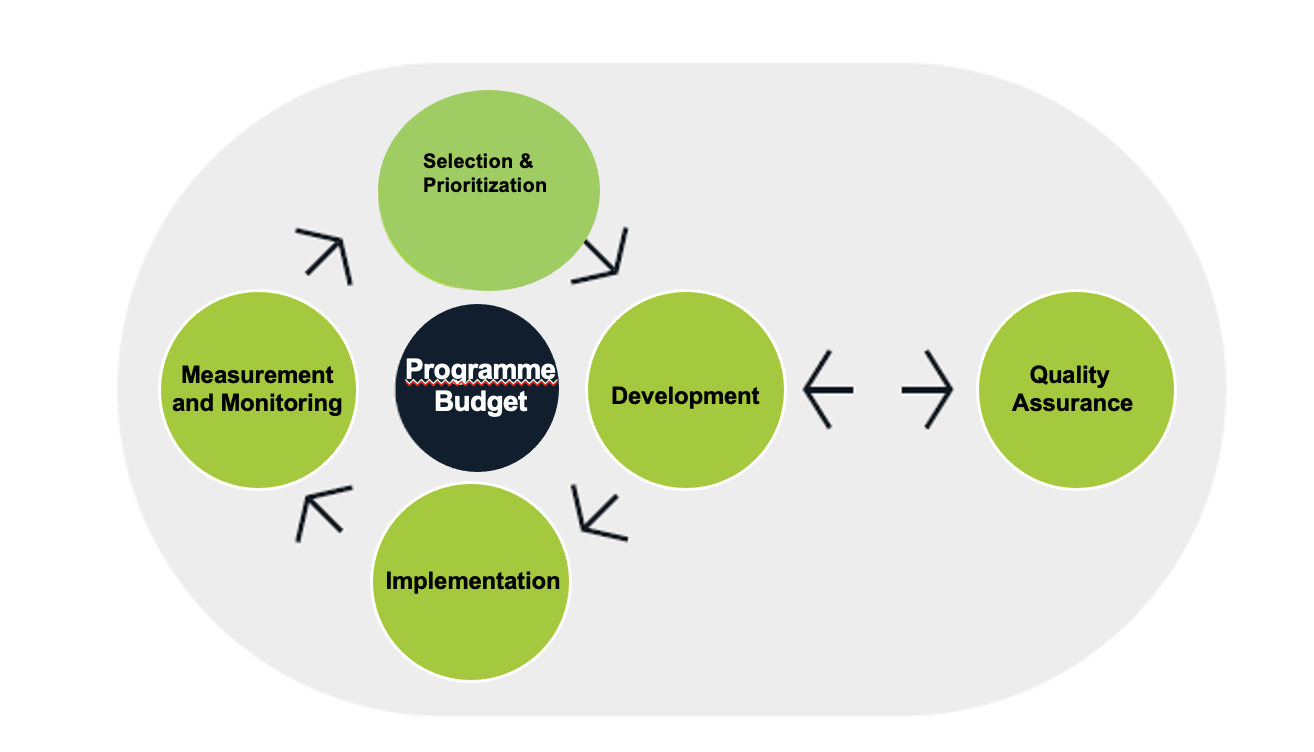
The TP lifecycle is a cross-Organizational process managed through 3 level Technical Expert Networks and Output Delivery Teams to ensure coherence across the five lifecycle phases, maximizing country impact as the utmost priority.
Technical Products (TPs) must fill a need that is not already filled by an existing product, which is a mandatory criteria, AND also meet at least one of the following criteria:
- Responds to country needs
- Is called for in a Governing Body resolution
- Is linked to evidence-based emerging global or regional public health issues and/or reflects demonstrable need based on evidence from the public health community.
The Office of the Deputy Director General (DDGO) provides oversight and coordination of the Technical Products (TPs) process and, in collaboration with Planning, Resource Coordination and Performance Monitoring (PRP), Quality Assurance of Norms and Standards (QNS) and Data, Analytics and Delivery for Impact (DDI), has made the downloadable list of GPHGs and TPs electronically accessible to support countries as they respond to addressing the health needs of the population.
